In the realm of chemistry, the concept of mole measurement stands as a cornerstone, providing a precise and universal language for quantifying the composition and behavior of substances. Join us as we delve into the intricacies of mole measurement, exploring its applications and unraveling its significance in various scientific fields.
Mole measurement enables chemists to determine the number of atoms, molecules, or ions present in a given sample, empowering them to understand the stoichiometry of chemical reactions and make accurate predictions about the behavior of substances.
Mole Measurement
The mole, denoted by the symbol mol, is a fundamental unit of measurement in chemistry. It represents a specific amount of a substance, defined as the quantity that contains exactly 6.02214076×10 23elementary entities. These entities can be atoms, molecules, ions, or electrons.
Examples of Mole Usage
Moles are used extensively in chemical calculations to determine the amount of reactants and products involved in a reaction. For instance, if we know the number of moles of a reactant, we can calculate the mass of the reactant or the volume of a gas produced.
Relationship with Other Units
The mole is related to other units of measurement, such as mass and volume. The molar mass of a substance is its mass per mole, expressed in grams per mole (g/mol). The molar volume of a gas is its volume per mole, typically expressed in liters per mole (L/mol).
Mole Calculations
Calculating Moles from Mass, Mole measurement
To calculate the number of moles in a given mass of a substance, we divide the mass by the molar mass. For example, to find the number of moles in 100 g of sodium chloride (NaCl), we divide 100 g by 58.44 g/mol, which gives us 1.71 mol of NaCl.
Stoichiometry
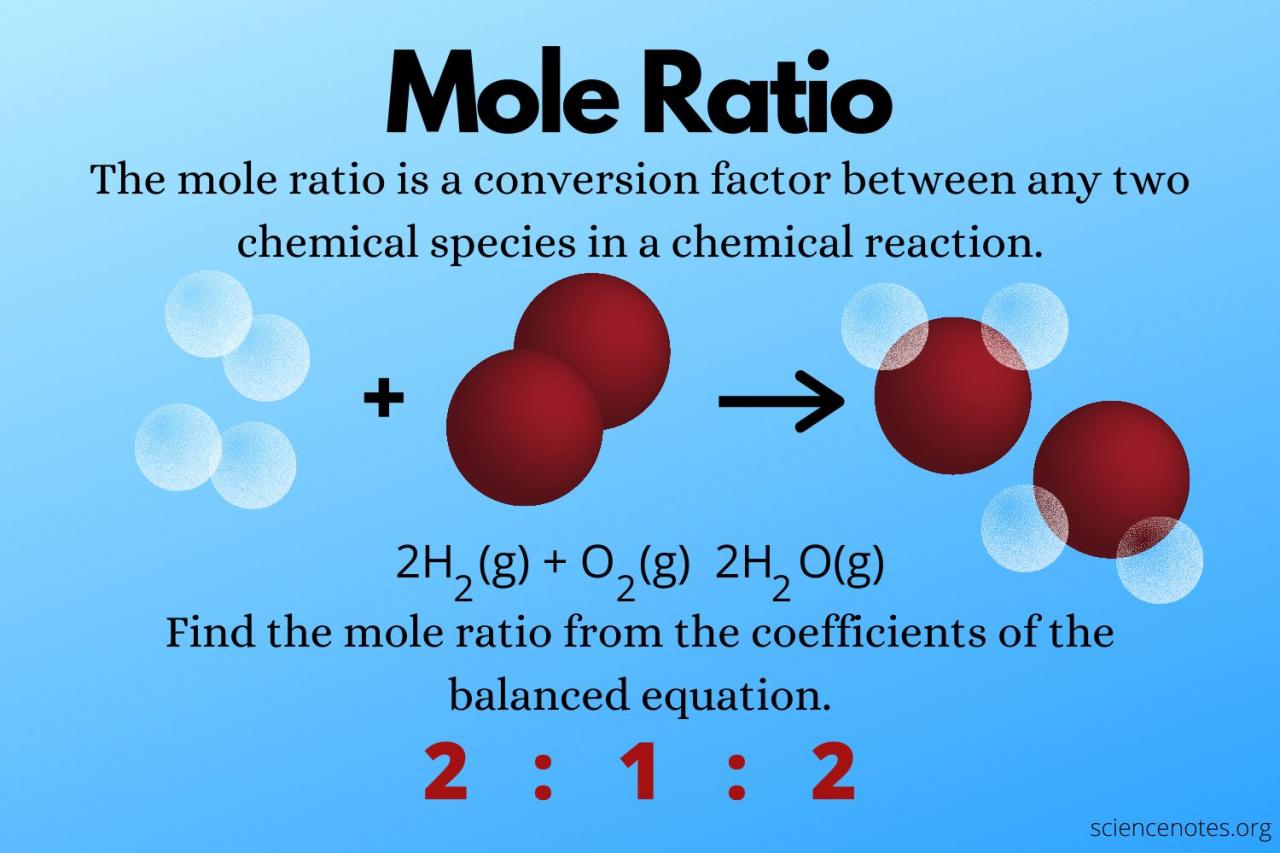
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Using stoichiometry, we can determine the moles of reactants and products involved in a reaction based on the balanced chemical equation.
Molarity and Concentration
Definition of Molarity
Molarity is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution. It is expressed in units of moles per liter (mol/L).
Calculating Molarity
To calculate the molarity of a solution, we divide the number of moles of solute by the volume of the solution in liters. For example, if we have 0.5 moles of sodium chloride dissolved in 1 liter of water, the molarity of the solution is 0.5 mol/L.
Comparison with Other Concentration Units
Molarity is often compared to other concentration units, such as molality and normality. Molality is the number of moles of solute per kilogram of solvent, while normality is the number of equivalents of solute per liter of solution.
Mole Conversions: Mole Measurement
Converting between different units of measurement using moles is essential in chemistry. The following table summarizes the most common mole conversions:
| Conversion | Formula |
|---|---|
| Mass to moles | Moles = Mass / Molar mass |
| Moles to mass | Mass = Moles × Molar mass |
| Volume to moles (gas) | Moles = Volume / Molar volume |
| Moles to volume (gas) | Volume = Moles × Molar volume |
Applications of Mole Measurement

Mole measurement has wide-ranging applications in various fields:
- Medicine:Calculating drug dosages, determining blood glucose levels, and understanding enzyme reactions.
- Environmental Science:Monitoring air and water pollution, assessing greenhouse gas emissions, and analyzing soil composition.
- Materials Science:Designing new materials, optimizing manufacturing processes, and understanding the properties of substances.
Final Summary
Mole measurement has revolutionized our understanding of chemistry, providing a standardized and precise method for quantifying and manipulating matter. Its applications extend far beyond the laboratory, impacting fields as diverse as medicine, environmental science, and materials engineering. As we continue to explore the molecular world, mole measurement will undoubtedly remain an indispensable tool, enabling us to unravel the secrets of chemistry and unlock new frontiers in scientific discovery.
Popular Questions
What is the significance of mole measurement in chemistry?
Mole measurement provides a standardized and precise method for quantifying the composition and behavior of substances, enabling chemists to understand the stoichiometry of chemical reactions and make accurate predictions about the behavior of substances.
How is mole measurement used in real-world scenarios?
Mole measurement finds applications in various fields, including medicine, environmental science, and materials engineering. For instance, in medicine, it is used to determine the dosage of drugs and monitor the concentration of substances in the body.
What is the relationship between moles and other units of measurement?
Moles are related to other units of measurement such as mass and volume through Avogadro’s number, which represents the number of atoms, molecules, or ions present in one mole of a substance.